The Emerson Enhancement Effect
In 1957, Robert Emerson conducted experiments regarding what wavelengths most efficiently drive photosynthesis. He noticed that photosynthesis dramatically drops off at 680nm and above. This was considered strange since chlorophyll isolated in a beaker absorbs light well above this point. Photosynthesis reductions above 680nm became known as the “red drop effect”.
This led Emerson to additional experiments showing that plants respond disproportionately to the combination of red light and far-red wavelengths. He observed a dramatic increase in photosynthetic rates when the plants he tested were exposed to red and far-red light at the same time. This phenomenon became known as the “Emerson Enhancement Effect”.
It’s a non-linear function as the sum of the individual contributions is greater than the individual contributions themselves. It's almost like one plus one equals three.
But was it an enhancement? Since sunlight has almost far-red as red and plants evolved under the sun, it makes more sense that adding far-red to the spectra makes up for a deficiency, not an enhancement.
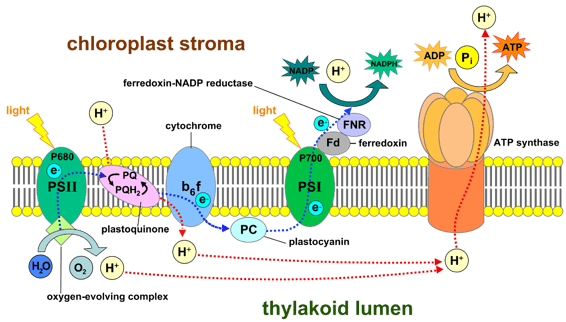
(Source: https://en.wikipedia.org/wiki/Light-dependent_reactions#/media/File:Thylakoid_membrane_3.svg By Somepics - Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=38088695)
So how does this work? Red light is absorbed by the PSII reaction center. This light energy is used to split up water molecules capturing an electron in the process. Fun fact: this is why plants “generate” oxygen. After two water molecules are split there is a resultant O2 molecule formed, and the photons are available for the plant to use in other processes.
2 H2Oà O2 + 4H+ + 4e-
The red light exposure also excites these electrons into a higher energy state. As the electron moves through the electron transport chain to PSI it does “work” such as facilitating the movement of a proton (H+) from the stroma to the lumen. This causes the electron to lose energy along the way. Once they hit the PSI reaction center, far-red light takes over and re-excites the electrons to provide the energy needed to reduce NADP+ to NADPH.
This is why far-red light is important. It's analogous to a booster pump. Plants can grow without it but it enables higher photosynthetic rates. Either it takes longer to move the electrons through the PSI reaction center, or a “more costly” process is used to get the same job done.
Now the next question is how much far-red? There can be some negative effects of too much far-red. Most of the red light that is exposed to plants is absorbed. Far-red light can penetrate through the leaves. When plants sense abundant far-red compared to red, they stretch as they feel that they are in the shade. This is an attempt to outgrow other plants that are causing the shading.
Another potential issue with too much far-red is it tends to make flowers “larfy” and airy. Most growers are trying to grow hard, dense flowers so keep the red to far-red ratio in check.
This post is dedicated to the late Dr. Marc van Iersel. His research was in horticultural physiology including photosynthesis, LED lighting, and optimization of controlled environment agriculture. He will be missed.
Tags:
Oct 6, 2023 9:00:00 PM


Comments